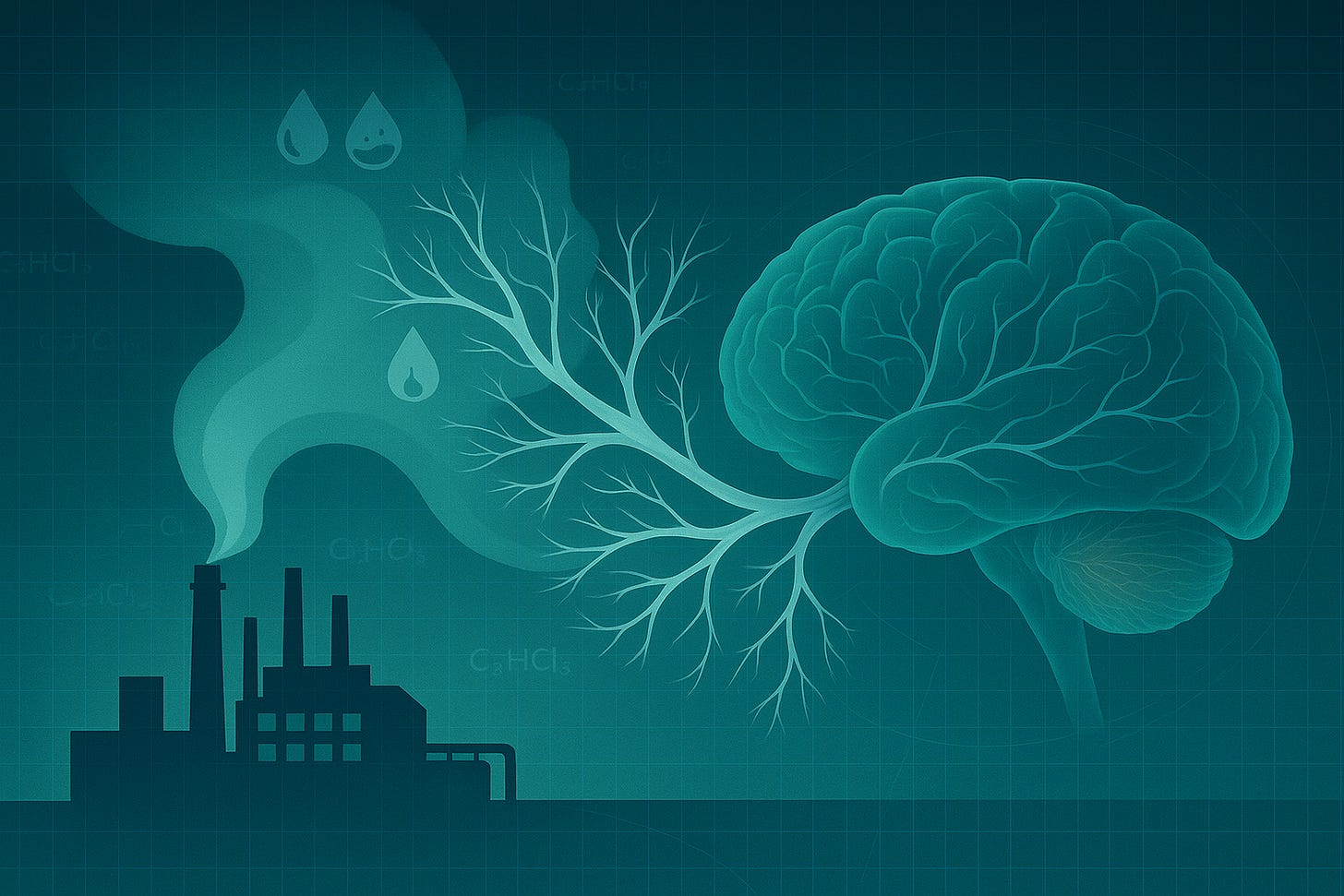
Trichloroethylene exposure increases Parkinson’s risk
Long-term exposure to trichloroethylene, a widely used industrial solvent, is now linked to a higher likelihood of developing Parkinson’s disease.
A new nationwide study suggests that long-term exposure to the industrial degreasing chemical trichloroethylene (TCE) may slightly raise the risk of Parkinson’s disease. While the increase in individual risk is modest, the widespread presence of TCE in air, soil, and water means that the potential population impact could be substantial.
Study Details
Rese…
Keep reading with a 7-day free trial
Subscribe to Just Healthcare to keep reading this post and get 7 days of free access to the full post archives.