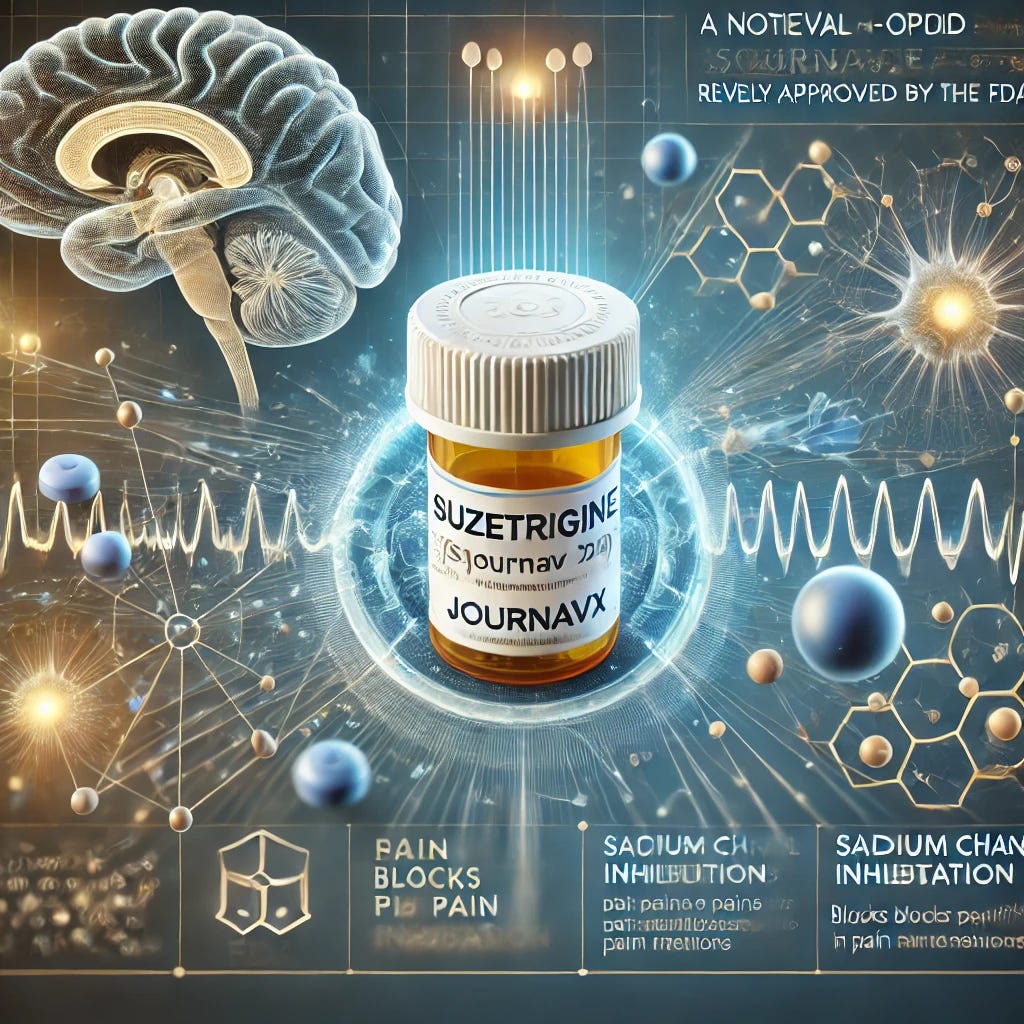
Non-Opioid Pain Pill for Acute Pain Relief Approved
Suzetrigine Offers a Novel Alternative to Opioids but Raises Questions About Efficacy
Topline
The FDA has approved suzetrigine (Journavx), the first non-opioid analgesic of its kind, for treating moderate to severe acute pain in adults. The drug, developed by Vertex Pharmaceuticals, is a highly selective NaV1.8 sodium channel blocker, targeting peripheral pain neurons without acting on the brain, making it a non-addictive alternative to o…
Keep reading with a 7-day free trial
Subscribe to Just Healthcare to keep reading this post and get 7 days of free access to the full post archives.