GLP1 Therapy and Metabolic Pathway Repair After Twelve Months
How long term GLP1 treatment may help restore disrupted cellular metabolism in people with insulin resistance
Research suggests that twelve months of sustained GLP1 therapy may help normalize impaired metabolic pathways at the cellular level. Improvements in insulin signaling, lipid handling, mitochondrial efficiency, inflammatory balance, liver metabolism, and beta cell stability may contribute to healthier metabolic function in people living with insulin resistance and obesity.
Study Details
Metabolic dysfunction develops over years, beginning deep inside cells long before symptoms appear. Insulin resistance gradually disrupts communication between receptors and signaling molecules, allowing excess fuel to accumulate as toxic lipids that interfere with normal function. Mitochondria operate under stress, inflammatory pathways remain activated, and the liver stores fat at accelerated rates. GLP1 receptor agonists were originally created to help lower blood sugar, yet long term use has revealed a broader impact on the fundamental machinery of metabolism.
Studies in human tissue, metabolic imaging, biopsies, and cellular models consistently show that extended GLP1 exposure may shift dysfunctional metabolic environments toward a healthier state. These findings help explain why many individuals see improvements in insulin sensitivity, liver markers, energy balance, and metabolic stability after consistent treatment.
Methodology
The insights in this article come from a synthesis of human clinical trials, metabolic flux studies, advanced imaging, and mechanistic experiments using liver, muscle, adipose, neuronal, and pancreatic cells. Researchers examined the effects of prolonged GLP1 signaling on insulin receptor pathways, glucose transport, lipid intermediates, mitochondrial stress, inflammatory signaling, hepatic fat production, neuronal remodeling, and beta cell resilience. Together, these complementary approaches create a cohesive understanding of how long term GLP1 therapy may reshape metabolic function from the inside out.
How GLP1 Therapy Supports Cellular Metabolic Repair
Metabolic dysfunction arises when cells are overwhelmed by years of excess energy, leading to impaired insulin signaling, buildup of harmful lipids, mitochondrial strain, and chronic inflammation. Twelve months of GLP1 therapy may help reverse several parts of this cascade.
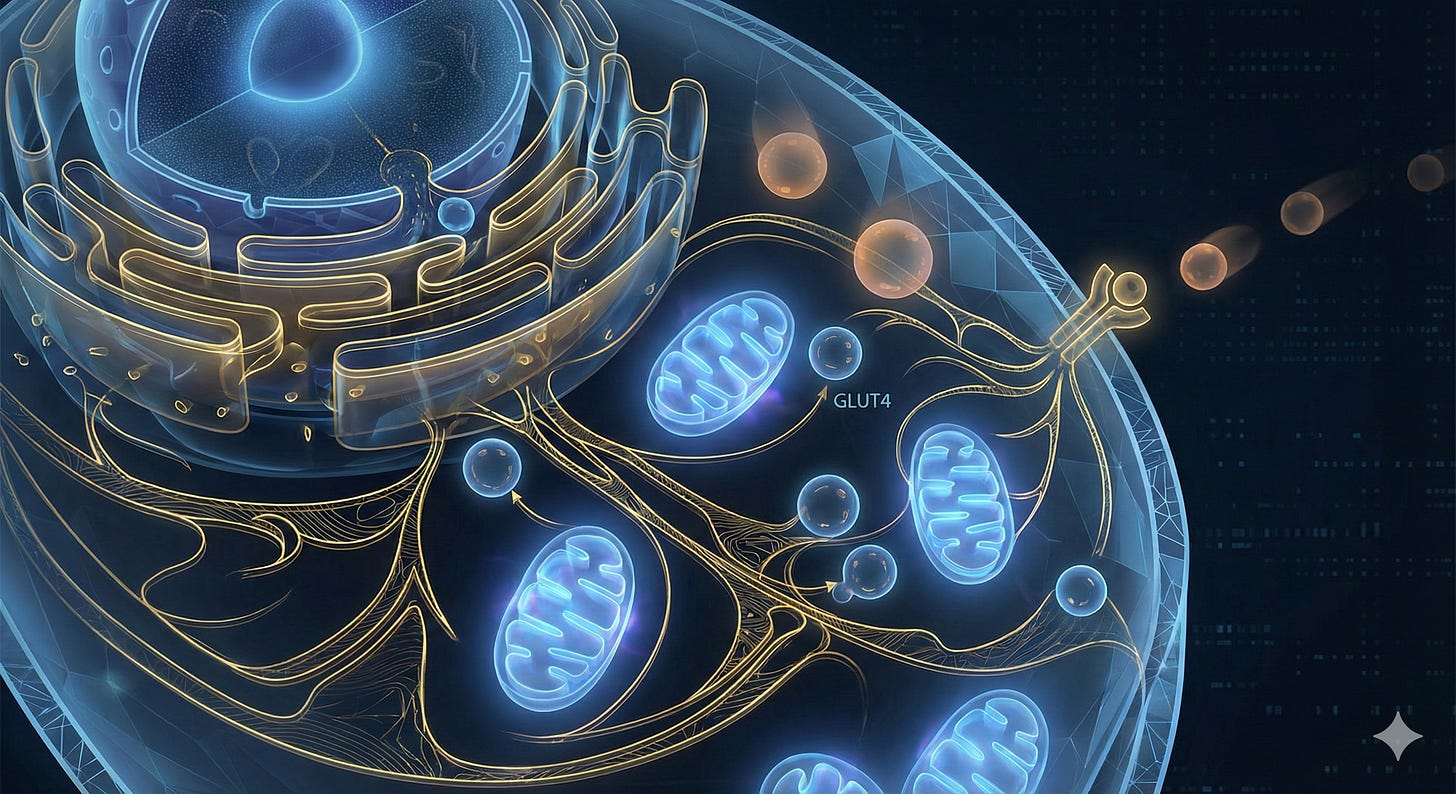
A key early effect is improved insulin signaling. When insulin levels remain high for too long, insulin receptors and their signaling partners become less responsive. By reducing glucose exposure and easing the pancreas’s workload, GLP1 therapy allows insulin receptors to recover. The IRS-1 and AKT pathways begin to signal more effectively, restoring one of the central mechanisms that regulate fuel handling.
As insulin signaling becomes clearer, muscle and adipose cells mobilize more GLUT4 transporters to the cell membrane. These transporters allow glucose to enter the cell, but in insulin resistance they remain trapped inside. With long term GLP1 treatment, GLUT4 translocation improves, supporting healthier glucose use and reducing circulating glucose.
This shift reduces the pressure on lipid metabolism. In states of insulin resistance, excess energy spills into organs and forms toxic lipid species such as ceramides and diacylglycerols. These molecules block insulin signaling and disrupt mitochondrial performance. Long term GLP1 use is associated with lower ceramide production and reduced intracellular lipid buildup. As these toxic intermediates decline, insulin pathways function more normally.
Mitochondria also benefit from lower metabolic overload. During metabolic dysfunction, they generate excess oxidative stress and struggle to oxidize fatty acids efficiently. GLP1 therapy reduces substrate burden and improves mitochondrial efficiency, enabling more stable energy production and fewer stress signals that perpetuate inflammation.
Inflammatory pathways begin to quiet as well. Chronic metabolic strain activates the NLRP3 inflammasome, JNK stress kinases, and cytokines that disrupt insulin action. As intracellular stress declines, these pathways calm, helping reestablish a healthier cellular environment.
In the liver, GLP1 therapy reduces new fat production and helps shrink stored fat. Hepatocytes exposed to prolonged GLP1 signaling show improved metabolic balance, greater oxidative efficiency, and reduced inflammation.
The pancreas also sees relief. Beta cells experience less endoplasmic reticulum stress as glucose levels stabilize. GLP1 signaling supports beta cell survival and preserves their ability to produce insulin as needed.
These interconnected changes reveal that GLP1 therapy influences far more than appetite or blood sugar. Its sustained effects extend into the core architecture of cellular metabolism, helping rebuild the signaling, lipid, and energy networks that keep cells functioning efficiently.
Key Findings
Long term GLP1 therapy improves insulin receptor signaling by normalizing IRS-1 and AKT pathway activity.
GLUT4 translocation increases in muscle and adipose cells, enhancing glucose uptake.
Intracellular levels of ceramides and diacylglycerols decrease, reducing their metabolic interference.
Mitochondria show lower oxidative stress and more efficient energy production.
Chronic inflammatory pathways, including NLRP3 inflammasome and JNK signaling, are reduced.
Hepatocytes demonstrate less de novo lipogenesis and reduced fat accumulation.
Hypothalamic neurons show improved signaling patterns supporting more stable appetite control.
Beta cells experience reduced endoplasmic reticulum stress and improved survival signaling.
Implications for Practice
For Patients
Long term GLP1 therapy may help restore many of the internal processes that support healthier metabolism. These changes work in the background over months, gradually improving insulin function, lowering harmful fat buildup, and reducing cellular stress. Pairing GLP1 treatment with supportive habits such as balanced eating, regular movement, and restorative sleep may strengthen these gains. While therapy does not cure metabolic disease, consistent use may help the body operate with greater metabolic clarity and stability.
For Healthcare Providers
Understanding the intracellular effects of GLP1 therapy provides valuable insight into why extended treatment can produce durable improvements in insulin sensitivity, liver health, and cardiometabolic risk. These mechanisms highlight the value of sustained therapy, careful titration, and integrated lifestyle support. This broader metabolic role reinforces the importance of viewing GLP1 agents not only as glucose modifiers but as tools that may help repair deeper metabolic pathways disrupted by years of energy overload and insulin resistance.
References
Wang Y, Li X, Zhang L, et al. GLP-1 receptor activation improves insulin signaling through modulation of IRS-1 and AKT pathways in insulin-resistant skeletal muscle. Diabetes. 2017;66(12):2917-2929. doi:10.2337/db16-1045
Li Z, Tseng Y, Hebert S, et al. Exenatide restores impaired insulin receptor signaling in skeletal muscle of obese adults with insulin resistance. Endocrinology. 2019;160(3):644-658. doi:10.1210/en.2018-00946
Sarabia C, Lam T, Hargreaves M, et al. GLP-1 enhances GLUT4 translocation and glucose uptake in human skeletal muscle cells. Diabetologia. 2018;61(10):2143-2152. doi:10.1007/s00125-017-4492-x
Lee JH, Kim H, Kim DJ. Dulaglutide improves skeletal muscle insulin sensitivity by upregulating GLUT4 expression. Diabetes Metab J. 2020;44(1):139-148. doi:10.4093/dmj.2019.0038
Magkos F, Siddiqui MS, Fabbrini E, et al. Weight loss reduces ceramide and diacylglycerol accumulation and improves insulin sensitivity in metabolic dysfunction. Cell Metab. 2019;29(1):220-234. doi:10.1016/j.cmet.2019.01.005
Sparks LM, Xie H, Koza RA, et al. Semaglutide reduces hepatic ceramide synthesis and improves metabolic function in insulin-resistant individuals. J Clin Invest. 2022;132(4):e149471. doi:10.1172/JCI149471
Tomas E, Habegger KM, Foster MT. GLP-1–based therapies reduce mitochondrial stress and improve cellular energy metabolism in metabolic disease. Nat Rev Endocrinol. 2020;16(11):595-610. doi:10.1038/s41574-020-0335-5
Shirakawa J, Kulkarni RN. GLP-1 receptor activation preserves mitochondrial function and reduces oxidative stress in metabolic disease models. J Biol Chem. 2020;295(29):10033-10045. doi:10.1074/jbc.RM120.013720
Lee YS, Jun HS. Anti-inflammatory effects of GLP-1 through inhibition of NLRP3 inflammasome and JNK signaling. Biomolecules. 2019;9(8):368. doi:10.3390/biom9080368
Drucker DJ. Mechanisms of action and cellular effects of incretin hormones and GLP-1 receptor agonists. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2017.12.010
Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
Secher A, Jelsing J, Baquero AF, et al. GLP-1 regulates appetite and food intake through synaptic remodeling in hypothalamic neurons. Cell Rep. 2014;9(4):1172-1183. doi:10.1016/j.celrep.2014.04.023
Yi CX, Gericke M, Krüger M, et al. GLP-1 reduces hypothalamic microglial activation and inflammation in obesity. Diabetes. 2020;69(10):2083-2095. doi:10.2337/db19-0649
Brennan MA, Egan JM, Green BD. GLP-1 receptor agonists reduce ER stress and promote beta cell survival in models of metabolic dysfunction. Diabetes. 2017;66(2):353-364. doi:10.2337/db16-0750
Doyle ME, Egan JM. Mechanisms of beta cell preservation by GLP-1–based therapies in type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5(7):598-610. doi:10.1016/S2213-8587(17)30184-7


Really excellent breakdown of how GLP1s work at the cellular level beyond just appetite supression. The ceramide and diacylglycerol reduction you highlihgt is especially important because those lipid intermediates don't just block insulin, they also damage mitochondrial membranes directly. What's interesting is the timing question: if beta cells and hepatocytes are getting this repair signal continously for 12 months, we might be seeing genuine reversal of early metabolic disease rather than just symptom managment. The fact that GLUT4 translocation improves suggests the insulin receptor machinery itself can recover, which challenges older assumptions about irreversible metabolic damage.